Health
AstraZeneca Removes Covid-19 Vaccine from the UK Market
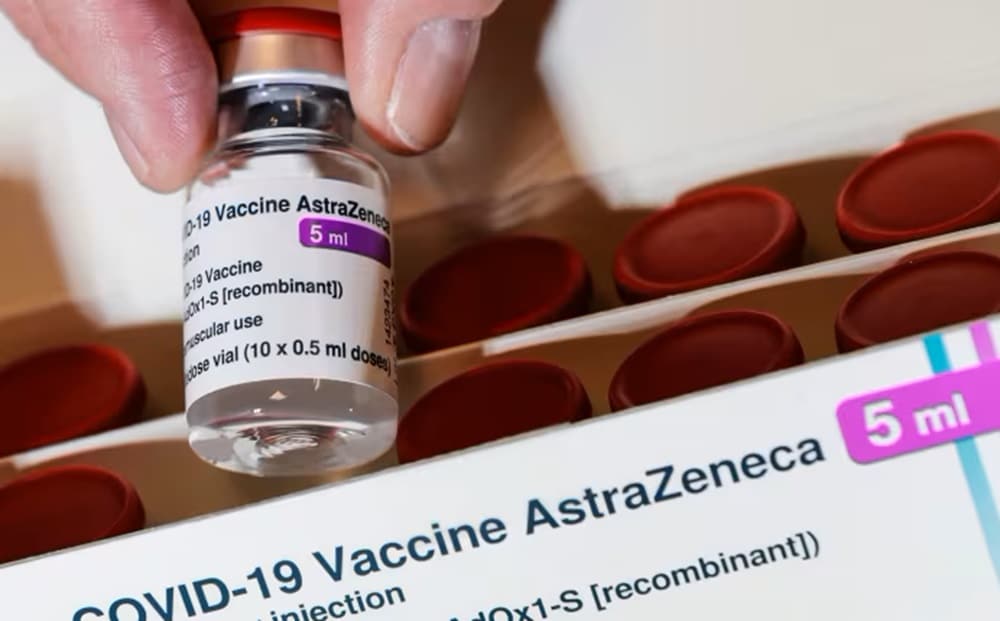
AstraZeneca is pulling its Covid-19 vaccine from the UK market less than four years after its debut, citing a “surplus” of vaccines targeting newer strains and declining demand.
On Wednesday, AstraZeneca stated that while it was “proud of the role Vaxzevria played in ending the global pandemic,” the company would no longer manufacture or supply the medicine due to a “surplus of available updated vaccines.”
The decision marks the end of the road for the vaccine, which was developed in partnership with experts at Oxford University within months of the pandemic’s breakout. It was authorized in the UK in late 2020, and over 3 billion doses have been distributed since its debut.
Unlike rivals Pfizer, BioNTech, and Moderna, AstraZeneca initially used a non-profit approach for its vaccine, selling it “at cost” as part of an agreement with Oxford. The medication was critical in ending the epidemic. However, its deployment was marred by rare cases of blood clotting and disagreements with the European Union over access to medicine.
“According to independent estimates, over 6.5 million lives were saved in the first year of use alone,” AstraZeneca stated, adding that additional COVID-19 vaccines have since been produced, reducing sales of its own medicine.
First Vaccine Approved in the UK
The announcement comes after the pharmaceutical company sought in March that the European Union withdraw its marketing authorization for Vaxzevria, which was granted on Tuesday.
AstraZeneca’s vaccine has been supplanted by mRNA-based vaccines produced by BioNTech/Pfizer and Moderna, which are now the most widely used worldwide.
According to the company’s full-year figures, AstraZeneca’s jab generated only $12 million in sales in 2023, compared to nearly $4 billion in 2021. In late 2021, AstraZeneca signed its first for-profit arrangements, claiming the pandemic had entered an “endemic phase.”
The vaccine was approved in the United Kingdom in December 2020 and the European Union in January 2021, but it was never approved in the United States, where authorities criticized the company’s presentation of data on vaccination efficacy.
Overall, the vaccination was safe and effective, but confidence in it dipped in 2021 following a string of rare blood-clotting occurrences, prompting European authorities to restrict its use among younger people.
Jamie Scott is suing the firm, alleging that taking the vaccine caused him to develop a major blood clot. If held accountable, the UK government’s vaccine damage payment plan would compensate for any damages. The business stated that the removal was unrelated to the uncommon blood clotting incidences.
AstraZeneca stated: “We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
About AstraZeneca
AstraZeneca is a global pharmaceutical corporation based in Cambridge, England. It develops and manufactures various medications to treat various medical ailments. During the COVID-19 epidemic, the business earned headlines for its collaborative efforts to create a vaccine with Oxford University.
Vaxzevria COVID-19 vaccine was one of the first vaccines approved for emergency use worldwide. Despite initial issues with efficacy data and worries about potential adverse effects, the vaccination proved successful in preventing severe illness and death from COVID-19. It was essential in vaccination campaigns throughout Europe and the rest of the world.
Their line of pharmaceuticals extends beyond the COVID-19 vaccine to include cancer, cardiology, respiratory, and metabolic illnesses. The corporation invests substantially in R&D, hoping to bring breakthrough therapies to market. It operates in over 100 countries and employs tens of thousands worldwide.
AstraZeneca has experienced numerous controversies and legal challenges, including litigation involving drug pricing and marketing activities. However, it remains a key player in the pharmaceutical sector, strongly emphasizing scientific research and global health programs. The company’s response to the COVID-19 epidemic has strengthened its position as a major contributor to global public health efforts.
Source: The Financial Times
Health
Kelly Clarkson Weight Loss Wasn’t Ozempic It Was a High Protein Diet
Kelly Clarkson’s remarkable weight loss has been a major topic of conversation for quite some time now, and the 42-year-old singer and talk show presenter has been very open about it with her fans!
The Kelly Clarkson program host had spoken up about her ever-shrinking figure multiple times, including on her talk program, when she admitted to utilizing a weight loss injection (not Ozempic!) to help her owing to being pre-diabetic.
Kelly revealed that she has lost a lot of weight, saying, ‘Mine is a different one than people assume, but I ended up needing to do it also because my blood work was so poor.’ She said that she had not taken Ozempic.
Kelly Clarkson did not name the medicine but described it as “something that aids in the breakdown of the sugar—my body does not do it right.”
She said her doctor ‘chased [her] for, like, two years’ to take the medication, but she was concerned about the consequences on her thyroid. However, she took it after seeing a birthday special she intended to release.
Kelly Clarkson Weight Loss
‘All of a sudden I halted it, and I was like, “Who the f*ck is that?'” she added. “You see it and you’re like, “Well, she’s about to die of a heart attack”,” Kelly said.
Whoopi, for her part, said she shed the weight of ‘nearly two people’ after ‘taking that great shot that works for persons who need some help.’
“It’s great for people like us who have issues,” the View co-host continued. She mentioned earlier that she is using Mounjaro for weight loss.
Her weight loss began following a health concern.
Kelly Clarkson originally hinted at her weight loss on her talk show, The Kelly Clarkson Show, in December 2023. According to US Today, she previously stated that she no longer wore Spanx. “It’s quite cold inside this building. I don’t even have to wear Spanx anymore. “I just wear them for warmth, like thermals,” Kelly explained during a singing game.
However, on January 29, Kelly said on her show that she was doing ‘ something’ about her weight after obtaining a pre-diabetic diagnosis a few years prior.
(According to the Centres for Disease Control and Prevention, pre-diabetes means having blood sugar levels that are ‘greater than usual’ but not high enough for a type 2 diabetes diagnosis.) ‘I wasn’t astonished,’ she explained. ‘I was a little bit overweight.
‘They said, “You’re pre-diabetic.” You’re right on the brink.” And I was like, “But I’m not there yet,” she added. ‘And then I waited two years and said, “Okay, I’ll do something about it.”‘
High Protein Diet
Kelly Clarkson has changed her diet and is focussing on consuming plenty of protein.
‘I eat a healthy mix,’ she told People. ‘I lost weight because I listened to my doctor, which I hadn’t done in a few years. And I succeed 90% of the time since a protein-rich diet already benefits me. I’m a Texas gal, so I enjoy meat—sorry, vegans of the world!”
Kelly stated that her diet is a ‘healthy mix’, which means she still allows sweets.
I still indulge. ‘The other night, I had frozen yoghurt with my daughter, and it was fantastic,’ she continued.
Kelly stated that in 2018 when on a weight-loss journey, she would change the ingredients in her meals to make them healthier. ‘It’s the same stuff you eat; I use different ingredients,’ she explained.
‘Even for fried chicken, I use cassava flour, tapioca, or almond flour, while you use hormone-free chicken.’
However, Kelly agreed that this is not the most convenient option for most people. ‘I’m going to be honest with you: it’s incredibly expensive,’ she said.
Kelly later stated that she had lost weight after reading Dr. Steven Gundry’s The Plant Paradox. The Plant Paradox Diet is lectin-free, excluding beans, legumes, whole grains, some vegetables, and dairy.
‘I literally read this book, and I followed it for an autoimmune condition and a thyroid issue, and now all of my numbers are back up,’ Kelly told Extra the same year. ‘Thanks to this book, I’m no longer taking medication. It’s all about how we cook our food: non-GMO, pesticide-free, and eating organically.
In addition to nutrition and exercise, Kelly revealed that she has been using infrared saunas, which have been shown to promote sleep, ease tension and pain, and help clarify skin.
She’s also tried cold plunges. ‘I just took a chilly plunge because everyone wore me down,’ Kelly explained.
Related News:
7 Causes of Eye Bags: Why You Look Tired
Health
MAID Now Accounts for 1 in 20 Deaths in Canada
Medical assistance in dying (MAID), often known as voluntary euthanasia, accounted for 4.7% of Canadian deaths in 2023, according to new Health Canada data.
According to Heath Canada’s fifth annual report, since the Trudeau government legalized MAID in 2016, about 15,300 persons will undergo assisted death in 2023 if their applications are granted.
The median age in this group was more than 77. The great majority, almost 96%, died from “reasonably foreseeable” causes, such as cancer.
In a tiny number of other cases, patients may not have been terminally sick but wanted assisted suicide owing to a protracted and difficult illness that had significantly reduced their quality of life.
Canada is one of a few countries that have passed assisted dying legislation in the last decade. Others include Australia, New Zealand, Spain, and Austria.
In Canada, consenting adults can request medical help in dying from a healthcare physician if they have a serious and irreversible medical condition. Some constraints exist, such as requiring two independent healthcare providers to certify the patient’s eligibility before the request is authorized.
Quebec Highest in MAID Deaths
In 2023, more than 320,000 individuals died in Canada, with medical assistance accounting for 15,300 of those fatalities (or around one in every 20).
According to estimates presented by Health Canada on Wednesday, the rate of assisted dying in Canada would rise by about 16% in 2023. This figure represents a significant decrease from the average increase of 31% in prior years.
The research stated that it is too early to determine what caused the rate to slow. For the first time, the report examined race and ethnic data on persons who received MAID.
Around 96% of receivers identified as caucasian, who comprise over 70% of Canada’s population. It’s unknown what produced the difference.
The second-highest reported ethnic group was East Asians (1.8%), who comprise approximately 5.7% of Canadians.
MAID remained the most commonly used method in Quebec, accounting for roughly 37% of all euthanasia fatalities despite the province’s population being only 22% of Canada.
The Quebec government initiated a study earlier this year to investigate why its euthanasia rate was so high.
Expanded Access to MAID
In 2021, the Trudeau government expanded access to MAID for persons who do not have a terminal diagnosis but wish to terminate their lives due to a chronic, disabling ailment. Earlier this year, it was announced that access to those with mental problems would be expanded again.
However, it was postponed for the second time because Canadian provinces, which control healthcare delivery, raised concerns about the system’s ability to handle such a large expansion.
On Wednesday, Health Canada defended the procedure, citing the criminal code’s “strict eligibility” conditions.
However, Cardus, a Christian research tank, claimed the latest MAID data were “alarming” and revealed that Canada has one of the world’s fastest-growing euthanasia regimes.
A report released in October by the Ontario government offered some insight on contentious cases in which people were awarded assisted dying when they were not reaching the end of their natural lives.
One example was a woman in her fifties with a history of melancholy and suicide ideation who was extremely sensitive to chemicals.
Her plea for euthanasia was granted after she was unable to find a home that could meet her medical requirements.
Another example made headlines recently, involving a Nova Scotia cancer patient who claimed she was twice asked if she was aware of assisted dying as an option while undergoing mastectomy surgery.
According to the National Post, the question “came up in completely inappropriate places”.
Many other Canadian news sites have also covered incidents in which people with disabilities considered assisted suicide owing to a lack of housing or disability assistance.
Related News:
BC Supreme Court Stops MAID Death of Woman from Alberta
Health
Diabetes Patients May Benefit From GLP-1 Medications
(VOR News) – Individuals with diabetes utilising GLP-1 medications, such as Ozempic or Mounjaro, may be gaining an additional benefit, as suggested by recent research findings. This advantage is a diminished probability of developing a potentially fatal blood clot.
The study’s findings revealed that diabetic patients on specific medications exhibited a twenty percent reduced risk of developing venous thromboembolism (VTE) compared to those on alternative diabetic treatments.
Dr. Rushad Patell, the principal author of the study, remarked that “from a public health perspective, considering the widespread use of these [GLP-1] drugs, there exists potential to ascertain whether the overall incidence of VTE could be diminished at a national or population level as a consequence of this study.”
This pertains to the prevalence of diabetes medications.
Given the escalating risk of venous thromboembolism (VTE), it is plausible that this will result in a shift of the curve in the contrary direction.
At the American Society of Haematology’s (ASH) annual meeting, which took place in San Diego on Sunday, his team gave a presentation of their research findings. The meeting took place in San Diego.
It is essential to keep these data in a preliminary form until they are published in a peer-reviewed publication because they were presented at a diabetes medical congress. At the convention, the results were presented.
The researchers highlighted that vein thromboembolism (VTE) is a prevalent clot formation in veins that can pose significant risks. The two predominant forms of venous thromboembolism are pulmonary embolism and deep vein thrombosis (DVT). Pulmonary embolisms are defined by the migration of blood clots to the lungs, whereas deep vein thromboses (DVTs) are defined by the formation of blood clots in the legs.
Any form of venous thromboembolism (VTE) can lead to hospitalisation and potentially death if left untreated.
Could the newly discovered GLP-1 diabetic medications, which have achieved significant market success, aid in the prevention of venous thromboembolism?
Over 558,000 individuals in the United States were registered in a comprehensive health care database, and Patell’s team monitored the outcomes of these participants to gather information regarding the circumstances.
Patients were categorised into two groups, each including roughly 279,000 individuals: those utilising a GLP-1 drug for diabetes control and those receiving an older class of diabetes medication referred to as DPP4i. Patients with comparable health conditions were divided into these two groups. DPP4 inhibitors, conversely, do not induce weight loss in the manner that specific GLP-1 medications do.
In comparison to the cohort receiving alternative diabetes treatment, the group administered GLP-1 therapy exhibited an average incidence of venous thromboembolism (VTE) of 6.5 per 1,000 patients after one year.
Clots per 1,000 patients in the alternative diabetes cohort were 7.9.
According to Patell and his colleagues, the risk of blood clot formation was diminished by twenty percent as a result of this. The occurrence of pulmonary embolisms and deep vein thromboses (DVTs) has been shown to be decreasing.
The researchers found that the patient’s obesity status before taking GLP-1 did not affect the advantages regarding clotting risk, which were consistent regardless of the individual’s weight. The ambiguity remains over whether the decreased clotting risk associated with GLP-1s is due to weight loss in individuals or if an alternative mechanism is involved. There is insufficient comprehension concerning this issue.
“Further studies are necessary to ascertain the potential mechanism, whether via weight loss or alternative methods,” Patell stated in a news release disseminated at an ASH convention: “We must identify the potential mechanism through weight loss.”
The study could not establish that the use of GLP-1s was the cause of the reduction in clotting due to its retrospective design. The study was conducted, which was the reason for this situation. Consequently, Patell and his associates have asserted that a prospective clinical trial is essential to validate the evidence reported to date. Patell asserts that the newly acquired data may still offer direction to individuals with diabetes and the medical experts who manage their care.
His hypothesis is that this finding implies potential advantages in choosing a GLP-1 receptor agonist as an antidiabetic drug for patients. He stated, “It is crucial to consider thrombotic risk when selecting an antidiabetic agent for a patient.”
SOURCE: USN
SEE ALSO:
Vesta Care Dubai: Transforming Healthcare with Personalized Home Services
Bird Flu Testing Mandatory For Milk Supply In Exclusive-US Issued Order
-
Politics3 weeks ago
Miller Expects 4.9 Million Foreigners to Leave Canada Voluntarily
-
News3 weeks ago
Nolinor Boeing 737 Crash Lands in Montreal
-
News2 weeks ago
“Shocking Video” Vancouver Police Shoot Armed Suspect 10 Times
-
Tech3 weeks ago
Increasing its Stake in OpenAI by $1.5 Billion is a Possibility for SoftBank.
-
News4 weeks ago
Facebook Securities Fraud Case Dropped
-
Health4 weeks ago
A Canadian Teenager’s Bird Flu Virus Has Mutations