Health
AstraZeneca Removes Covid-19 Vaccine from the UK Market
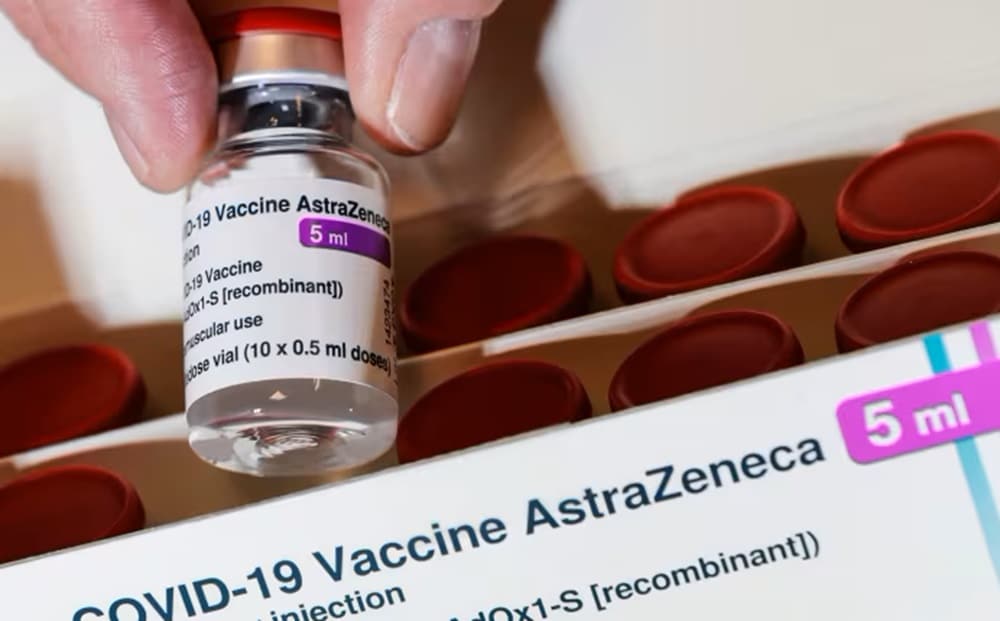
AstraZeneca is pulling its Covid-19 vaccine from the UK market less than four years after its debut, citing a “surplus” of vaccines targeting newer strains and declining demand.
On Wednesday, AstraZeneca stated that while it was “proud of the role Vaxzevria played in ending the global pandemic,” the company would no longer manufacture or supply the medicine due to a “surplus of available updated vaccines.”
The decision marks the end of the road for the vaccine, which was developed in partnership with experts at Oxford University within months of the pandemic’s breakout. It was authorized in the UK in late 2020, and over 3 billion doses have been distributed since its debut.
Unlike rivals Pfizer, BioNTech, and Moderna, AstraZeneca initially used a non-profit approach for its vaccine, selling it “at cost” as part of an agreement with Oxford. The medication was critical in ending the epidemic. However, its deployment was marred by rare cases of blood clotting and disagreements with the European Union over access to medicine.
“According to independent estimates, over 6.5 million lives were saved in the first year of use alone,” AstraZeneca stated, adding that additional COVID-19 vaccines have since been produced, reducing sales of its own medicine.
First Vaccine Approved in the UK
The announcement comes after the pharmaceutical company sought in March that the European Union withdraw its marketing authorization for Vaxzevria, which was granted on Tuesday.
AstraZeneca’s vaccine has been supplanted by mRNA-based vaccines produced by BioNTech/Pfizer and Moderna, which are now the most widely used worldwide.
According to the company’s full-year figures, AstraZeneca’s jab generated only $12 million in sales in 2023, compared to nearly $4 billion in 2021. In late 2021, AstraZeneca signed its first for-profit arrangements, claiming the pandemic had entered an “endemic phase.”
The vaccine was approved in the United Kingdom in December 2020 and the European Union in January 2021, but it was never approved in the United States, where authorities criticized the company’s presentation of data on vaccination efficacy.
Overall, the vaccination was safe and effective, but confidence in it dipped in 2021 following a string of rare blood-clotting occurrences, prompting European authorities to restrict its use among younger people.
Jamie Scott is suing the firm, alleging that taking the vaccine caused him to develop a major blood clot. If held accountable, the UK government’s vaccine damage payment plan would compensate for any damages. The business stated that the removal was unrelated to the uncommon blood clotting incidences.
AstraZeneca stated: “We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
About AstraZeneca
AstraZeneca is a global pharmaceutical corporation based in Cambridge, England. It develops and manufactures various medications to treat various medical ailments. During the COVID-19 epidemic, the business earned headlines for its collaborative efforts to create a vaccine with Oxford University.
Vaxzevria COVID-19 vaccine was one of the first vaccines approved for emergency use worldwide. Despite initial issues with efficacy data and worries about potential adverse effects, the vaccination proved successful in preventing severe illness and death from COVID-19. It was essential in vaccination campaigns throughout Europe and the rest of the world.
Their line of pharmaceuticals extends beyond the COVID-19 vaccine to include cancer, cardiology, respiratory, and metabolic illnesses. The corporation invests substantially in R&D, hoping to bring breakthrough therapies to market. It operates in over 100 countries and employs tens of thousands worldwide.
AstraZeneca has experienced numerous controversies and legal challenges, including litigation involving drug pricing and marketing activities. However, it remains a key player in the pharmaceutical sector, strongly emphasizing scientific research and global health programs. The company’s response to the COVID-19 epidemic has strengthened its position as a major contributor to global public health efforts.
Source: The Financial Times
Health
Cervical Cancer Patients Live Longer With New Drug Regimens

(VOR News) – Some encouraging developments have been made in the treatment of locally advanced cervical cancer. According to a new study, adding chemotherapy to normal treatment for a six-week period reduces the risk of death by about forty percent.
In a press release from Cancer Research UK, the organization that funded the study, Principal Investigator Dr. Mary McCormack of the University College London Cancer Institute said, “this represents the most significant advancement in outcomes for this disease in over two decades.”
“We have collected the requisite evidence to enhance the treatment of cervical cancer patients globally, and I am exceedingly proud of all the patients who participated in the trial.”
The new protocol reduces the risk of cervical cancer recurring by 35 percent.
This study, which was published this week in The Lancet, divided 500 patients from 32 medical facilities in Brazil, India, Italy, Mexico, and the United Kingdom into two groups using random assignment.
The inquiry was carried out from 2012 until 2022. There were no examples of cancer that had spread to other organs; all of the patients had locally progressed cervical cancer.
Chemoradiotherapy, a traditional treatment regimen consisting of radiation and cisplatin, was the only treatment given to the control group. The experimental group received chemotherapy with carboplatin and paclitaxel six weeks before receiving chemotherapy.
What details did they find out?
Forty-two percent of patients who underwent a restricted course of chemotherapy did not experience any metastasis or recurrence of the disease, while eight percent of patients received chemotherapy.
Eight percent of patients survived for at least five more years after starting treatment. Of those in the control group, 64% did not have any cancer metastases or recurrences, and 72% of them lived for at least five years.
According to a news statement from Cancer Research UK’s executive head of research and innovation, Dr. Iain Foulkes, “timing is paramount in cancer treatment.” He went on to say that the most important aspect of cancer treatment is time.
“This trial demonstrates that the simple incorporation of induction chemotherapy at the onset of chemoradiation for cervical cancer has yielded significant outcomes.”
“An increasing body of research is demonstrating the advantages of supplementary chemotherapy cycles prior to other interventions such as radiation and surgery in various cancers,” Foulkes told reporters. “It can be swiftly administered using medications that are currently widely available globally, and it may also reduce the probability of cancer recurrence.”
However, the course of treatment had a detrimental impact and produced unfavorable outcomes.
The majority of research participants suffered from a range of side effects, such as low white blood cell counts, infections, exhaustion, and gastrointestinal problems.
Compared to the 48% of patients who had chemotherapy and radiation therapy alone, 59% of patients who received their first chemotherapy reported side effects that were either extremely or potentially fatal or life-threatening.
Nowadays, the most common treatment for cervical cancer is chemotherapy based on cisplatin. It has been shown that this medication increases survival rates by 30 to 50%.
Some doctors prefer chemotherapy to surgical excision.
Dr. Otis Brawley, an oncology professor at Johns Hopkins University and the former chief medical officer of the American disease Society, told that “we comprehend that surgery will inevitably result in residual cancer.”
With chemotherapy and radiation therapy, you can completely destroy any pelvic cancer cells from your body. It is possible that in females, we will cause a long-lasting and total remission.
According to Brawley, cervical cancer—which is often brought on by particular strains of the human papillomavirus (HPV)—used to be the main cause of cancer-related death for American women. The landscape has completely changed with the advent of an HPV vaccine that serves as a prophylactic against cervical cancer.
Additionally, a new test that allows patients to collect their own vaginal samples at home to expedite the HPV screening procedure was approved by the US Food and Drug Administration in May.
According to Brawley, in most situations, the HPV vaccine or screening can be given before the problem manifests itself. “None of the 4,400 individuals who succumb to cervical cancer undergo annual screening.
SOURCE: USN
SEE ALSO:
The Stocks Of UnitedHealth Lead The Dow. How Should I Deal With Low Earnings?
Naloxone Is Becoming More Popular Among Bystanders For Preventing Overdoses.
Shares Dropped After Novavax Claimed The FDA Stopped Flu And Covid-Flu Vaccinations.
Health
Shares Dropped After Novavax Claimed The FDA Stopped Flu And Covid-Flu Vaccinations.

(VOR News) – Shares of Novavax fell sharply after the company said on Wednesday that the Food and Drug Administration had put a hold on the company’s application for a stand-alone flu vaccination and a combo injectable that targets both influenza and Covid.
This was the outcome of the announcement made by the corporation. The application that the company submitted has been placed on hold by the Food and Drug Administration (FDA), it was reported.
The biotechnology Novavax company’s stock had a sharp decline on Wednesday of almost twenty percent, signaling a serious decline.
The so-called clinical hold has been implemented because to a single patient who suffered from nerve damage following the combo injection during a phase two experiment that was finished in July of the prior year.
The problem was caused by Novavax injections.
A clinical hold is an order granted to a company to delay or terminate a potential clinical trial being carried out on a pharmaceutical product, according to the Food and Drug Administration (FDA). This particular order is known as a clinical hold.
The impact of the suspension on Novavax’s capacity to start phase three studies linked to those vaccines and to furnish information on those trials is not fully clear. Whether or not the suspension will have an effect is not made apparent.
According to the state of affairs, the biotechnology company seems to have experienced a setback as a result of this incident. The business is finding it difficult to introduce new treatments to the market as demand for its Covid vaccine is steadily declining globally.
The FDA decided to place a clinical hold on Novavax’s injectable and stand-alone flu vaccine. The firm has responded by stating that it is collaborating with the government to find a solution.
The FDA is the abbreviation for the Food and Drug Administration. Based on earlier trials of its COVID and flu vaccines, the business claimed there had been no safety concerns related to the kind of nerve harm the patient had experienced. The victim’s nerve injury was mentioned in this passage.
In the preceding statement, Novavax refers to the patient’s history of nerve damage.
While expressing its belief that there is no conclusive link between the vaccine and the patient’s nerve injury, Novavax has also said that it is making every effort to provide the FDA with further evidence as it works toward this conclusion.
Even though Novavax has stated that it does not think there is such an association, this is the case. Dr. Robert Walker, the chief medical officer of Novavax, stated in a press statement that the company’s objective is to accomplish.
“Our goal is to successfully resolve this matter and to start our Phase 3 trial as soon as possible,” Walker said. “We are committed to achieving this goal.” “Our goal is to achieve this goal as soon as possible.”
If you’re not interested in receiving mRNA doses from Pfizer and Moderna, you might want to look at the protein-based Covid vaccine offered by Novavax.
These vaccines use more advanced vaccine technologies to teach cells how to generate proteins that trigger an immune response against Covid. These immunizations are given when they are given. The vaccine developed by Novavax is an option that has several advantages, according to the public health authorities.
Conversely, the vaccine created by Novavax offers defense against the virus by employing protein-based technology. This method has been applied routinely for many years in the administration of shingles and hepatitis B vaccinations.
SOURCE: CNBC
SEE ALSO:
The Stocks Of UnitedHealth Lead The Dow. How Should I Deal With Low Earnings?
Naloxone Is Becoming More Popular Among Bystanders For Preventing Overdoses.
Mammogram Centers Must Now Inform Women About Their Breast Density. Here’s How It Could Affect You
Health
The Stocks Of UnitedHealth Lead The Dow. How Should I Deal With Low Earnings?

(VOR News) – The shares of UnitedHealth Group (UNH) have experienced a decline of more than nine percent since the beginning of trade on Tuesday.
Consequently, the Dow Jones Industrial Average, which is a weighted average that is based on price, has entered the zone of negative values.
Additionally, the health insurance company has reduced the upper end of its prediction for full-year profits in order to compensate for a high third-quarter earnings result.
This has occurred at the same time that the rate of price decline has been occurring.
The three months that concluded on September 30 brought in a total of $100.8 billion in revenue for UnitedHealth, representing a 9.1% increase in revenue compared to the same time in the previous year.
Over that time, UnitedHealth premiums rose 7.1% to $77.4 billion.
This was one of the factors that contributed to its expansion. Profits per share (EPS) climbed by 9% to $7.15 from the same quarter the previous year, which is a proportional rise in terms of percentage growth.
In a statement released today, Andrew Witty, the Chief Executive Officer of UnitedHealth, stated that “our ongoing expansion, which is well-positioned for the years ahead, is a direct result of the innovative products and responsive service provided by our employees, which are embraced by the entire health care community on a daily basis.”
“Our products are innovative, and our employees are attentive to our customers’ requirements,” Witty stated in the statement.
Analysts were pleasantly delighted by the outcomes of the third quarter financial report for the University of New Hampshire. To be more specific, Yahoo Finance reports that Wall Street forecasted the company to generate $99.3 billion in revenue and $7 in earnings per share.
In order to account for the “business disruption impacts” caused by the attack that took place earlier this year at UnitedHealth’s Change Healthcare subsidiary, the company changed its projection of the company’s total profits for the entire year.
Response to the data breach that occurred earlier this year led to the implementation of this step. Currently, the company anticipates that its profits per share will fall somewhere in the region of $27.50 to $27.75, with a lower high end of that forecast. An adjustment was made to its initial forecast, which was somewhere between $27.50 and $28.
The modification acknowledges UnitedHealth’s recognition of this truth.
If I have shares in UnitedHealth, should I keep them, sell them, or buy more of them? What is the most effective course of action to take?
According to the total return basis, the shares of UnitedHealth Group have gained by 16.4% as of the commencement of trading on Tuesday. This figure takes into account both the movement of prices and dividends earned by the company.
Only the total return is taken into consideration when calculating this increase. Wall Street continues to have a good attitude toward the company, despite the fact that the Dow Jones stock has not gained by the same 24.2% that the S&P 500 has.
As per the data provided by S&P Global Market Intelligence, the average target price that analysts have projected for shares of University of New Hampshire is $627.53.
In order to obtain the information that was obtained, these analysts provided it. When compared to the current levels, this represents a value increase of more than fourteen percent. Furthermore, the suggestion that the majority of individuals have for the blue chip stock is that it be recommended as a Strong Buy.
An organization that provides financial services, Truist Securities, is one of the businesses that has a more positive outlook on shares of The University of New Hampshire. In addition to having a buy rating on the stock, they have set their price target for the stock at $640.
Truist analyst David MacDonald asserts that UnitedHealth possesses “highly integrated, complementary platforms that benefit from meaningful scale, diversification, and robust capabilities.” MacDonald’s statement was made in reference to UnitedHealth’s capabilities.
Additionally, UnitedHealth possesses a sizable balance sheet and generates a substantial quantity of cash flow, which is another important advantage. It is interesting to note that he also asserts that “core trends remain solid” notwithstanding the impact of the data breach that occurred at Change Healthcare that occurred!
SOURCE: PFK
SEE ALSO:
Mammogram Centers Must Now Inform Women About Their Breast Density. Here’s How It Could Affect You
Naloxone Is Becoming More Popular Among Bystanders For Preventing Overdoses.
Ozempic-Fueled Slimming Is Blowing Up The Wedding Dress Industry
-
News2 weeks ago
The Biden Administration can go Ahead With Student Loan Forgiveness, Says a Federal Judge.
-
News2 weeks ago
Tesla Recalls 27,000 Cybertrucks Due To A Rearview Camera Issue
-
World2 weeks ago
Uber Hires Yandex Spinoff Ride-Hail and Autonomous Delivery With Avride
-
Tech2 weeks ago
Accenture and NVIDIA Collaborate to Enhance AI Implementation.
-
Tech2 weeks ago
Meta has started the Facebook Content Monetization Program.
-
Election News2 weeks ago
Chief Operating Officer Of Truth Social’s Parent Company Resigns











































